Jacques Robidoux, Ph.D.

Assistant Professor
Email: robidouxj@ecu.edu
Voice: 252-744-5909
Our work primarily embraces two areas of research:
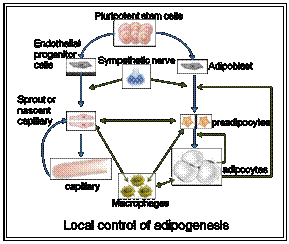
- We are investigating the autocrine/paracrine regulatory pathways that control the recruitment of new fat cells accompanying adipose tissue maintenance, remodeling and expansion in the adult.
- We are studying the molecular mechanisms that coordinate angiogenesis and adipogenesis during the course of adipose tissue remodeling and expansion.
We employ a combination of pharmacological, gain- and loss-of-function as well as genetic approaches. The models we are using or developing include human and mouse preadipocytes, human and mouse adipose explants, graft of engineered preadipocytes in athymic mice, adipose tissue knockout and adipose tissue specific transgenic mice.
Adipose tissue expansion is the combined result of the enlargement of existing fat cells and the recruitment of new adipocytes through proliferation, commitment and differentiation of precursor cells. These precursor cells that include mesenchymal stem cells, preadipocytes are cued by paracrine factors likely to be released from hypertrophied adipocytes, infiltrating macrophages, endothelial cells as well as neurotransmitters from nerve endings. As the adipocyte population grows, so does the vascular bed of the tissue. This occurs principally through sprouting of existing blood vessels and to a lesser extent trough differentiation of resident or blood born progenitor cells. Irrespective of the mechanisms involved, paracrine factors released from preadipocytes, adipocytes and macrophages seem to be orchestrating this remodeling of the adipose vasculature. Reciprocally, although ill defined, sprouting capillary might play a role in the growth promotion of the adipose lineage.
A deceptively straightforward anthropomorphic definition of obesity is that it constitutes an excessive accumulation of body fat. This, and the wealth of data showing that obesity contributes to the development of cardiovascular diseases and type II diabetes, gave adipose tissue its tarnished reputation. Noteworthy, and for a constellation of reasons, lipid accumulation in visceral rather than subcutaneous adipose tissues appears to portend the deleterious metabolic consequences. However, when comparing individuals with similar adipose mass, adipocyte size seems to be the dominant underlying risk factors for the development of the dyslipidemia and insulin resistance that underlies the development of above mentioned metabolic diseases.
Paradoxically, in both human and rodent models, excess or scarcity of adipose tissue is associated with an increased risk for most of the metabolic and cardiovascular disorders. Furthermore and intriguingly, in genetically engineered rodent models, increasing adipocyte number corrects most of the deleterious effect of both adipose tissue excess and deficiency. Therefore, the seemingly heretic idea that defects in adipose tissue expansion or remodeling could be a precipitating factor to the development of the metabolic syndrome.
Altogether, this underscores that a fundamental function of adipose tissue expansion is to safeguard against the deleterious consequences of a chronic positive energy balance. At this point the underlying mechanisms patterning a beneficial subcutaneous adipose tissue expansion is elusive and could be tentatively attributed to a prevention of ectopic fat accumulation and the release of contextually beneficial adipokines. We believe that this warrants for basic research on the molecular mechanisms of adipose tissue expansion.
Preliminary results from our laboratory suggest that the epidermal growth factor receptor (EGFR) collaborates with the IGF-1 receptor to promote preadipocyte proliferation, differentiation and lipogenesis.
Selected Papers
Kumar N., Liu D., Wang H., Robidoux J., Collins S. Orphan nuclear receptor NOR-1 enhances cAMP-dependent uncoupling protein-1 gene transcription. Revised version submitted to Mol Endocrinology on 12/28/2007
Wang H., Zhang Y., Yehuda-Shnaidman E., Medvedev A.V., Kumar N., Daniel K.W., Robidoux J., Mangelsdorf D.J., Collins S. Liver X receptor is a transcriptional repressor of the uncoupling protein-1 gene and the brown adipocyte phenotype. Revised version was submitted to Mol Cell Biol on 12/18/2007
Robidoux J., Simoneaux L., St-Pierre S, Ech-Hadli H., Lafond J. Human syncytiotrophoblast NPY receptors are located on BBM and activate PLC-to-PKC axis. Am J Physiol. 1998 Mar; 274(3 Pt 1):E502-E509.
Robidoux J., Pirouzi P., Lafond J., Savard R. Site-specific effects of sympathectomy on the adrenergic control of lipolysis in hamster fat cells. Can J Physiol Pharmacol. 1995 Apr; 73(4):450-458.
Book Chapters and Reviews
Collins S., Bai Y., Robidoux J. Chapter: Adipose Tissue Development and Metabolism. In Principles of Molecular Medicine. Second Edition, Humana Press. 2006
Collins S., Cao W., Robidoux J. Learning new tricks from old dogs: b-adrenergic receptors teach new lessons on firing up adipose tissue metabolism. Mol Endocrinology 2004; Sep;18(9):2123-2131
Robidoux J., Martin T.L., Collins S. b-Adrenergic Receptors and Regulation of Energy Expenditure: A Family Affair. Annu Rev Pharmacol Toxicol 2004; 44: 297-323
Collins S., Martin T.L., Surwit R.S., Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. 2004; Physiology and Behavior 2004 Apr;81(2): 243-8
Collins S., Cao W., Robidoux J., Daniels K. Mechanisms of βAR signaling in adipocytes and functional consequences on thermogenesis. 2003; Progress in obesity research 9: 135-138
Medvedev A.V., Robidoux J., Collins S. Molecular control of adipogenesis and obesity. The Investigational Drugs Journal. 2002 Feb; 5(2): 148-150
Laboratory Members
| Name | Title | Building/Room | Phone |
|---|---|---|---|
| Bin Luo | Research Specialist | BSOM 6S-26 | 252-744-2738 |